Notions of nomenclature for the use of the exercises on this site
The classification of organic compounds in the manner recommended by IUPAC requires you to make some remarks when there relates to a method for proposing a database-based exercises.
Alkyl residue "trivial names" such as tert-butyl or Isopropyl can create ambiguity, when you must assign a name to a molecule. In other words, can be used? Since it is not strictly "IUPAC expressions, when you want to build an educational system based on a database, you must make choices. The site database, can use these "trivial names". It will therefore be borne in mind that according to IUPAC, such groups may be appointed as 1-methylethyl (isopropyl) and 1,1-dimethylethyl (tert-butyl). You need so caution in assigning names. Please consider that, when you will find two or more substitutents whose name can be “trivial” you have two choices to build the whole molecular name. You can decide to use trivial or IUPAC names for those subsituents, but not both, in the same name. You can define a name having all substituents named by the trivial way or by IUPAC nomenclature system but not a mixture of the two methods.
As a second point, please remember that because often you write complex and long names, you have to pay attention to what you type on the keyboard. Often the correct typing can make all the difference.
Finally, as advice to Italian students who want to face with the nomenclature in the English language, which it is hoped since this is the nomenclature that is used in the chemical literature, you should put a lot of attention to the writing of the names, due to differences between the two languages (one for all: esano - hexane). For this purpose you can use as a reference this link.
Systematic Nomenclature
Rules for a Substitutive Nomenclature:
Systematic IUPAC Nomenclature
- Prefix (identity and position of substituents)
- Locants (numbers that define the position of substituents on the main, parent chain)
- Parent (Main chain of the molecule: based on its length as number of Carbon atoms)
- Suffix (based on the functional group having the highest priority, it defines the class of compounds to which to molecule belongs)
![]()
Alkanes
Step 1 - Identify the parent hydrocarbon chain
Step 1 - Identify the parent hydrocarbon chain
The main chain is the longest hydrocarbon chain that bring the functional group having the highest priority:
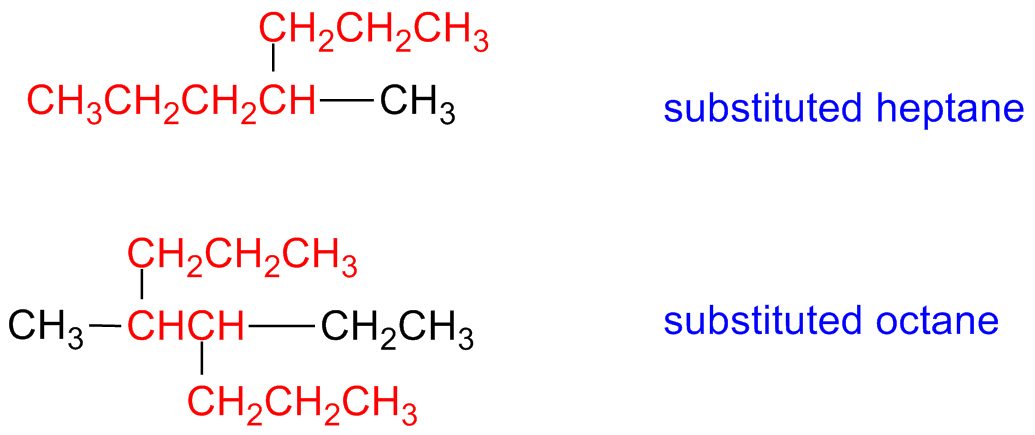
If there are more than one chain, showing the same number of carbon atoms,
the main chain is the chain having the highest number of branching.
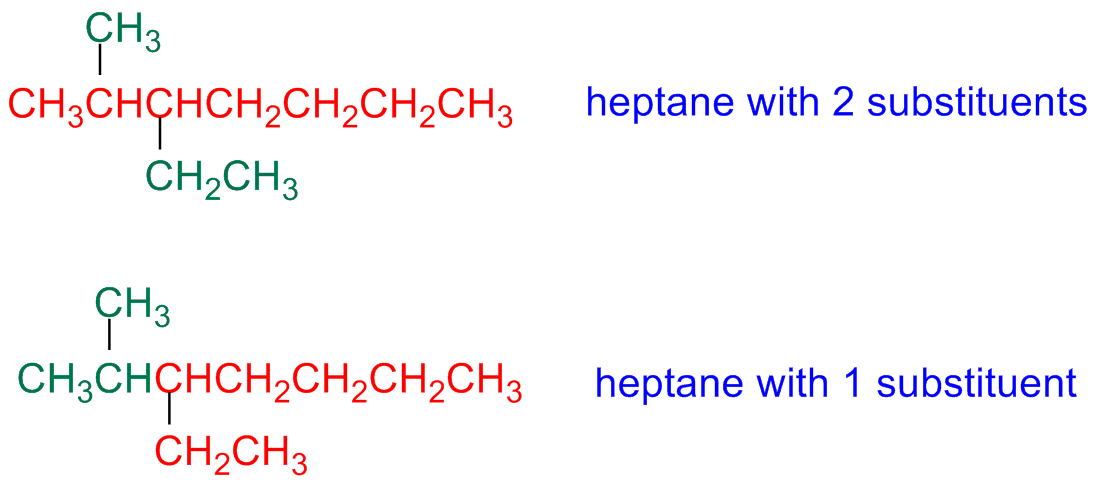
Step 2 - Number the parent chain carbon atoms
Step 2 - Number the parent chain carbon atoms
The parent chain carbon atoms are numbered
Start from the edge closest to the first branching point
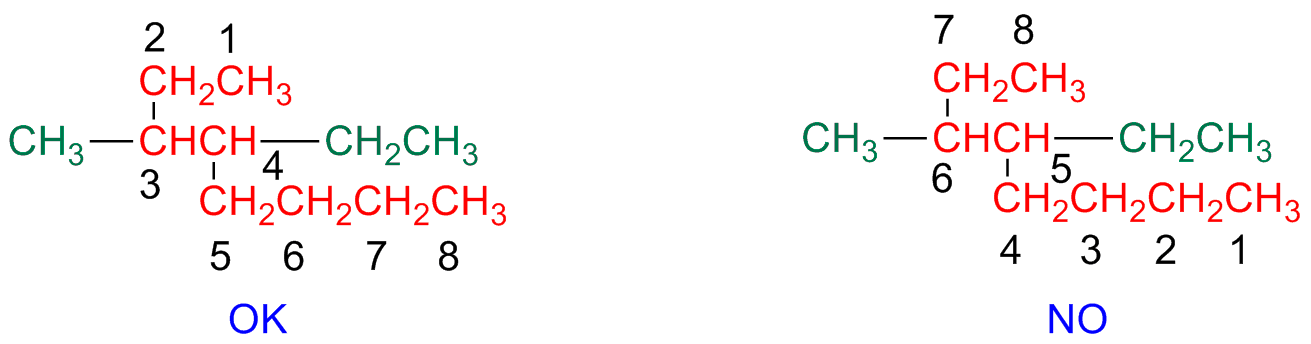
If the chain is branched at the same distance, starting from both edges, the numbering starts form the edge closest to the second branching point
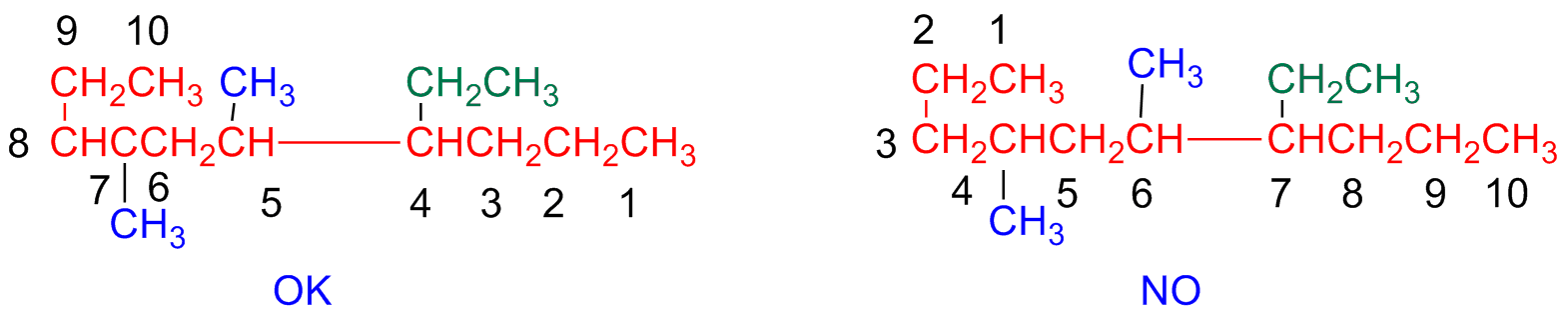
Step 3 - Identify and number substituents
Step 3 - Identify and number substituents
Substituents are identified and numbered
Every substituent is given a number based on its branching position on the main chain
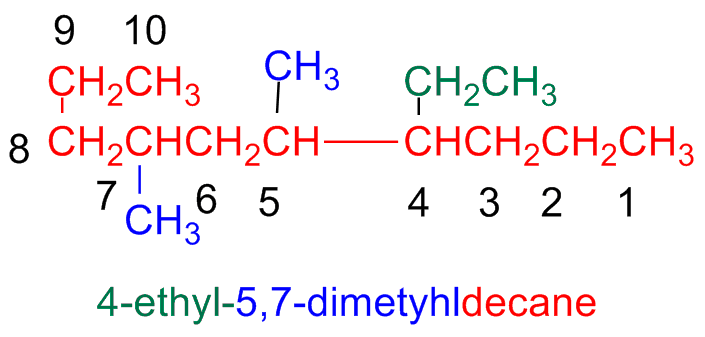
If on the same atom there are two substituents, each of them will have the same number
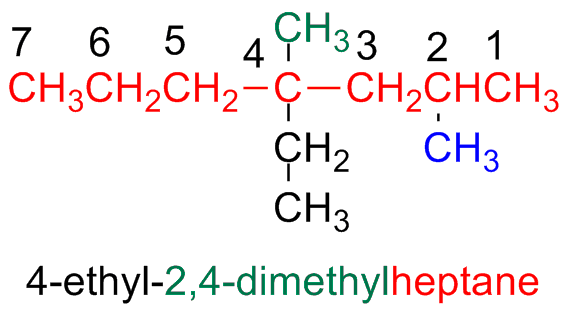
Step 4 - Write the name as a single word
Step 4 - Write the name as a single word
Write the name as a unique word
dashes (-) are used to separate different prefixes and commas (,) to separate numbers
If there are two or more side chains or substituents, they are written following the alphabetic order
If there are two or more equal side chains or substituents, numbering prefix are used like: di-, tri-, tetra, etc.
These numbering prefix are not considered for the alphabetic order
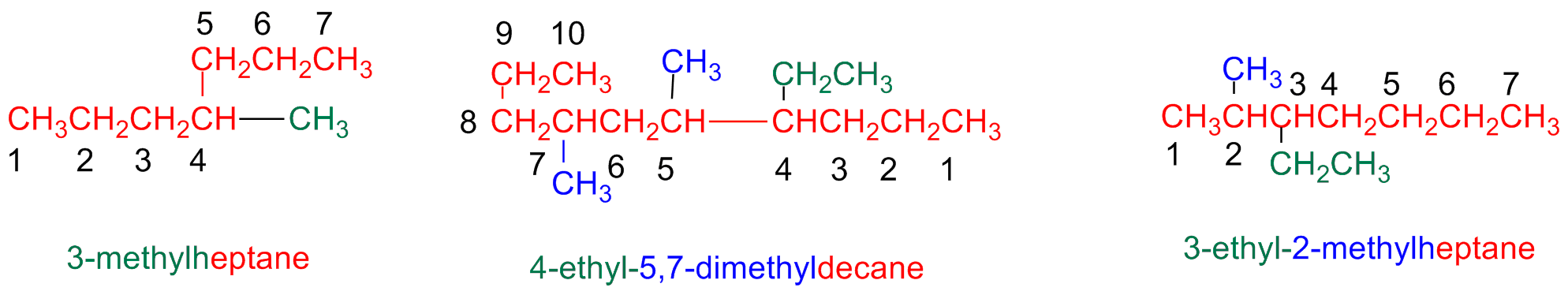
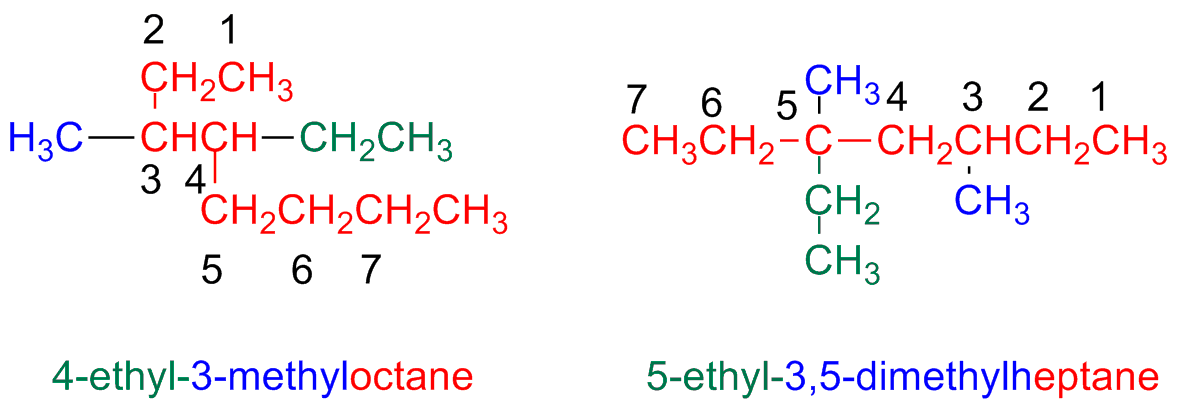
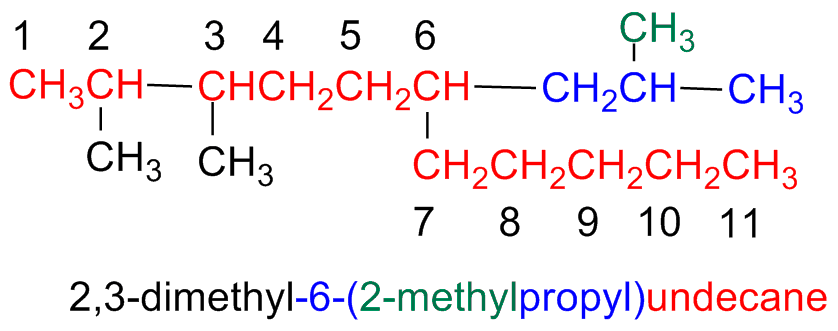
Step 5 – Give a name to a complex substituent as it were itself compound
Step 5 – Give a name to a complex substituent as it were itself compound
When a substituent is complex (i.e branched)...
Start numbering the components of the complex substituents starting form its attaching point the the main chain.
When writing the name, the substituent name is placed between parentheses a ordered following the alphabetic order. (numbering prefixes are taken into accounts)
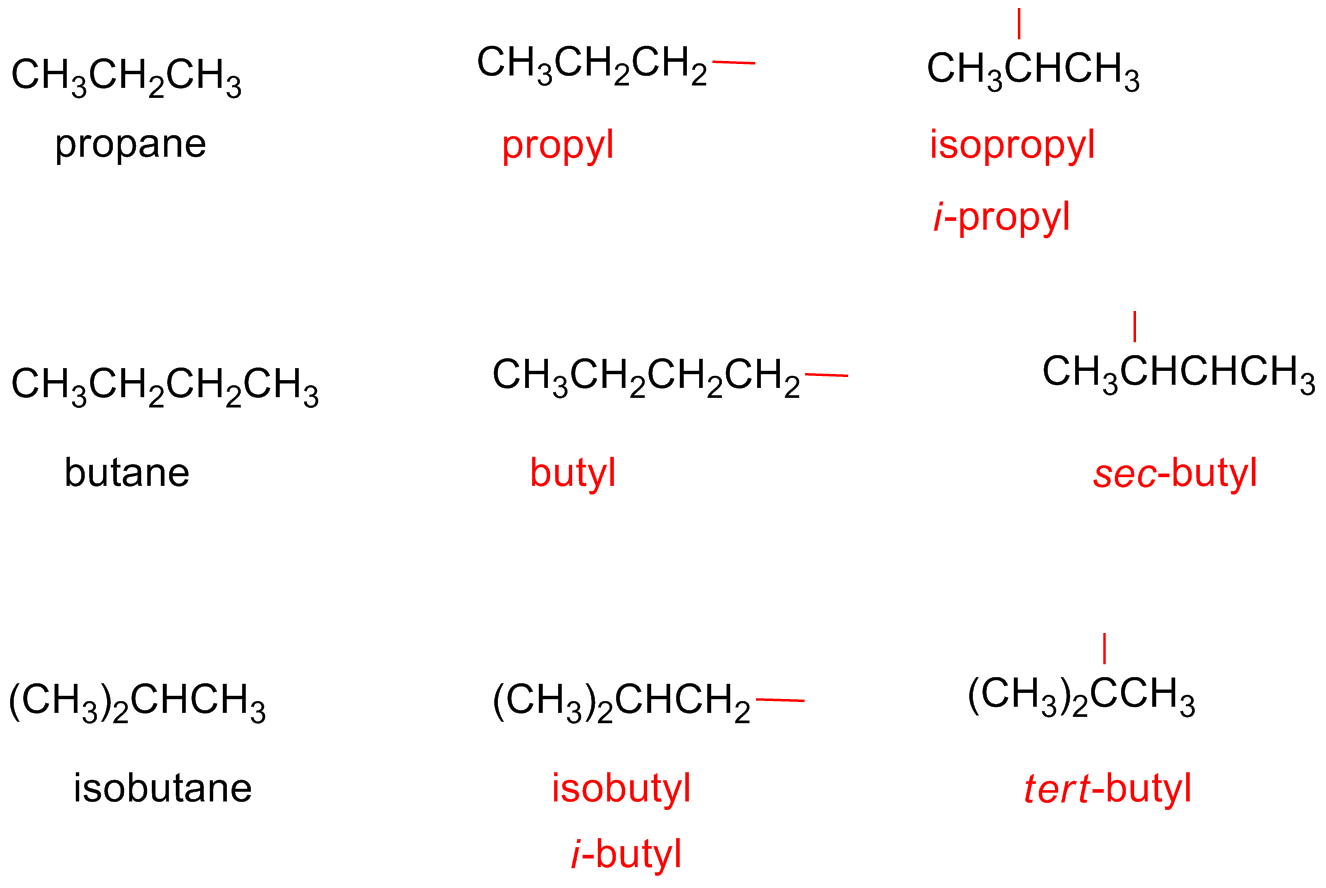
Alkyl Substituents
Alkyl Substituents
Alkyl Group or Alkyl Radical:
It is a partial structure which is derived form an alkane due to the extraction of an hydrogen atom.
Alkyl groups are named by substituting the suffix -ane with -yl.
Methane → Methyl
Ethane → Ethyl
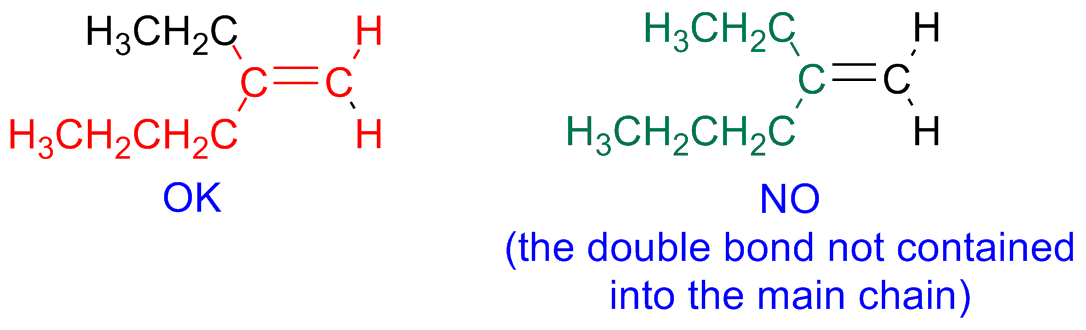
Alkenes
Step 1 - Identify the parent hydrocarbon chain
Step 1 - Identify the parent hydrocarbon chain
The main chain is the longest hydrocarbon chain that contains the double bond. The suffix is -ene:
Step 2 - Number the parent chain carbon atoms
Step 2 - Number the parent chain carbon atoms
Start form the edge closest to the double bond
If the double bond is at the same distance form both edges, start form the edge closest to the first branching point
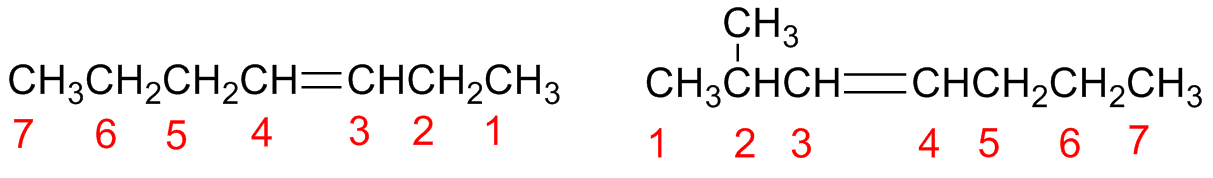
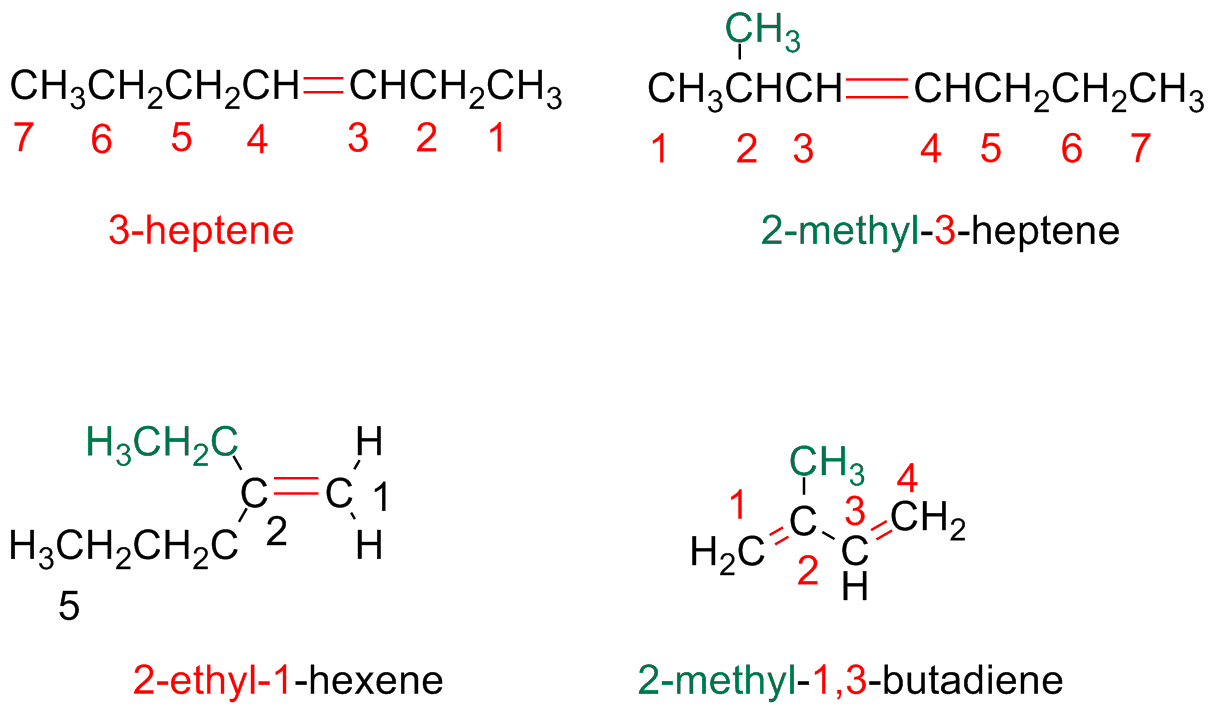
Step 4 - Write the name as a single word
Step 4 - Write the name as a single word
Number the substituents in the main chain and write them in alphabetic order.
The double bond is indicated with the number of its first carbon (smallest number).
This number is positioned just before the parent hydrocarbon chain which will have the suffix –ene.
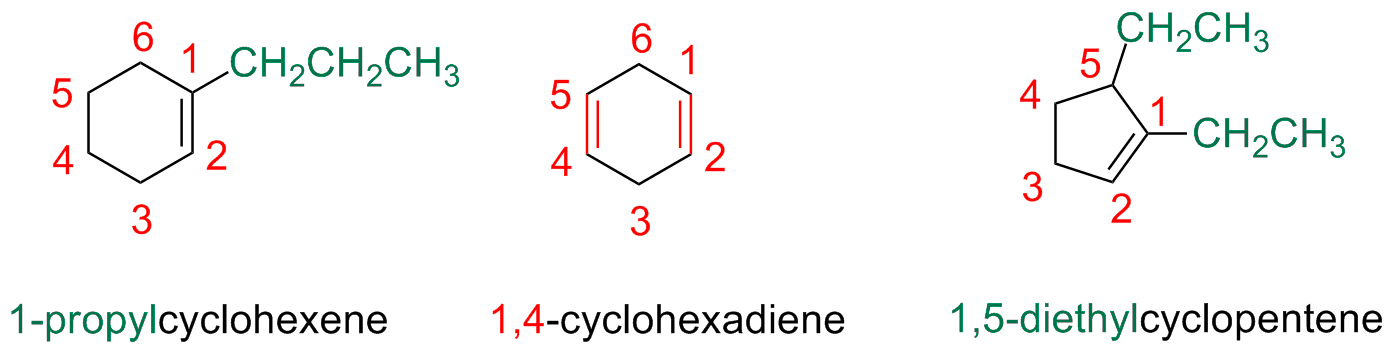
Cycloalkenes are numbered in a similar way
Start numbering by giving the smallest numnbber the alkene carbons (i.e 1 and 2)
The first substituent must have as low a number as possible
Alkynes
Step 1 - Identify the parent hydrocarbon chain
Step 1 - Identify the parent hydrocarbon chain
The main chain is the longest hydrocarbon chain that contains the triple bond
The tyriple bond position is indicated by the first carbon of the alkyne, the suffix is -yne.
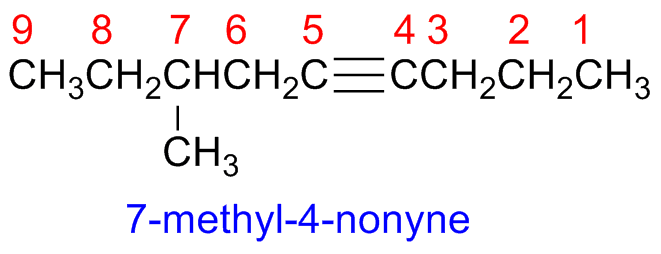
Step 2 - Number the parent chain carbon atoms
Step 2 - Number the parent chain carbon atoms
Number the parent chain carbon atoms
Start form the edge closest to the triple bond, and give it the smallest number as possible
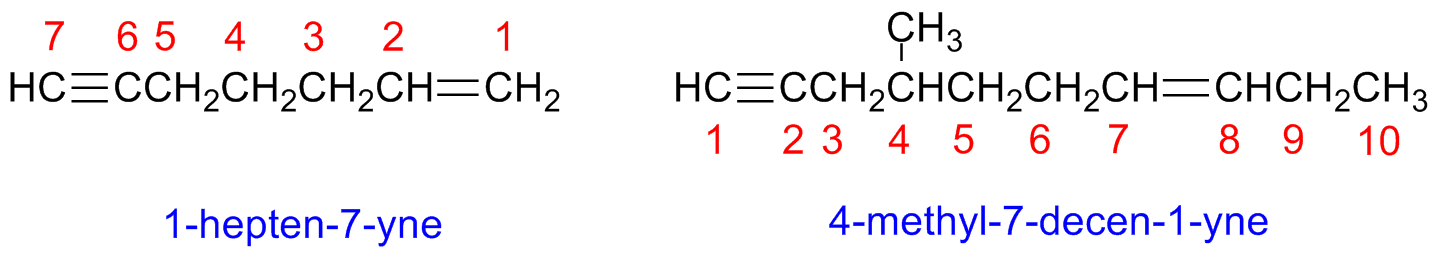
Compounds having more than a triple bond are named -diynes, -triynes, etc.
Compounds containing both double and triple bonds are named -enynes.
Start numbering enynes form the edge closets to a multiple bond (double or triple)
If both double and triple bonds could have the same number (i.e ambiguity in numbering), the double bond receive the lowest number
Alkyl, Alkenyl and Alkinyl Radicals
Alkyl, Alkenyl and Alkinyl Radicals
Substituents can contain multiple bonds:
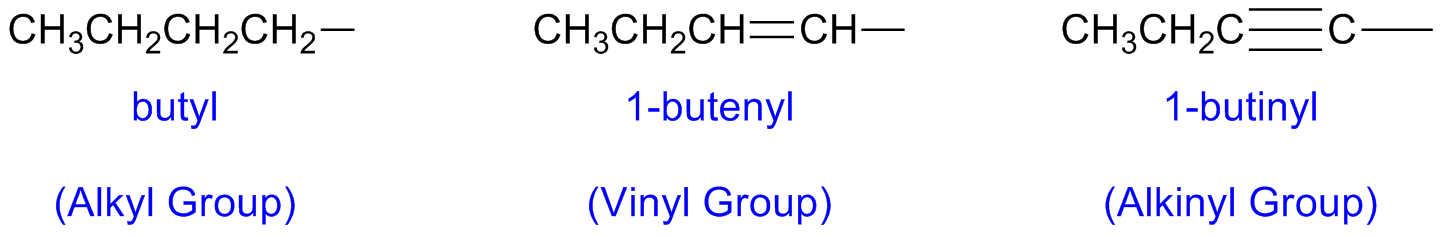
Alkyl Halides
Step 1 - Identify the parent hydrocarbon chain
Step 1 - Identify the parent hydrocarbon chain
Alkyl halides are numbers in the same way alkanes. The halogen atom is a substituent in the parent chain
If there are multiple bond, the parent chain must contain them
Step 2 - Identify and number substituents
Step 2 - Identify and number substituents
The main chain is numbered starting form the edge closest to the first substituent or branching point (i.e, alkyl or halide substituents have the same priority
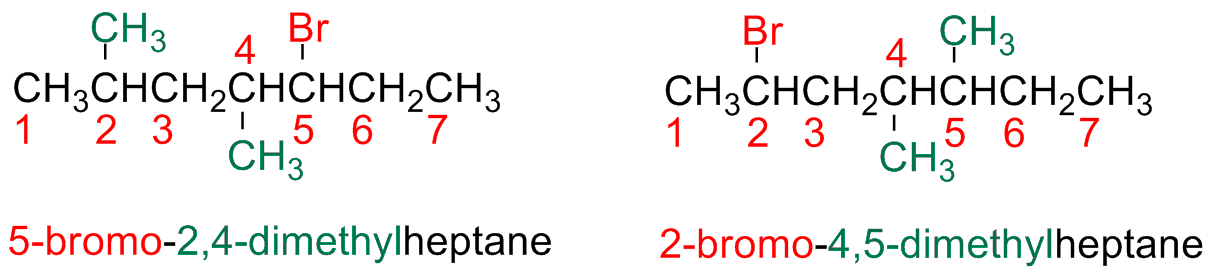
a) If there are more halogen atoms of the same nature, everyone of them is numbered and the prefix di-, tri-, tetra- etc. are used
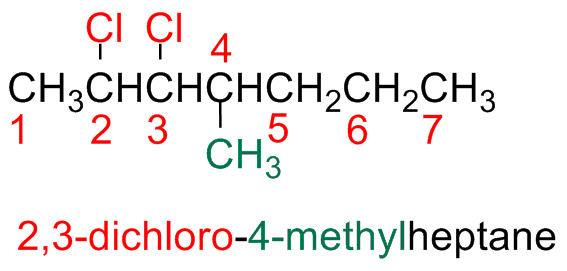
b) If there are more halogen atoms of different nature, all halogen atoms are numbered and they are indicated in alphabetic order
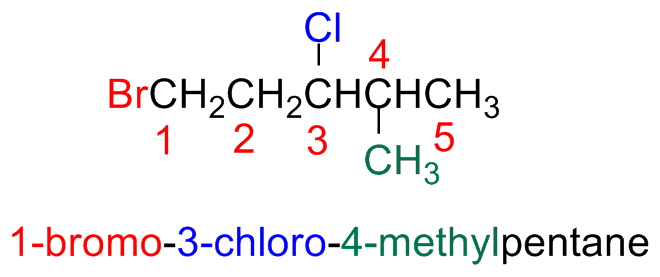
If one can start from both edges (step b), start form the edge closest start form the edge closest to the halogen (substituent) that comes first in alphabetic order
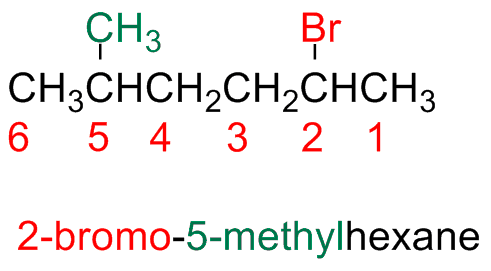
Aromatic Compounds
Generalities
Generalities
Often aromatics did not follow systematic nomenclature
Monosubstituted benzenes are named as other hydrocarbons, using the suffix –benzene
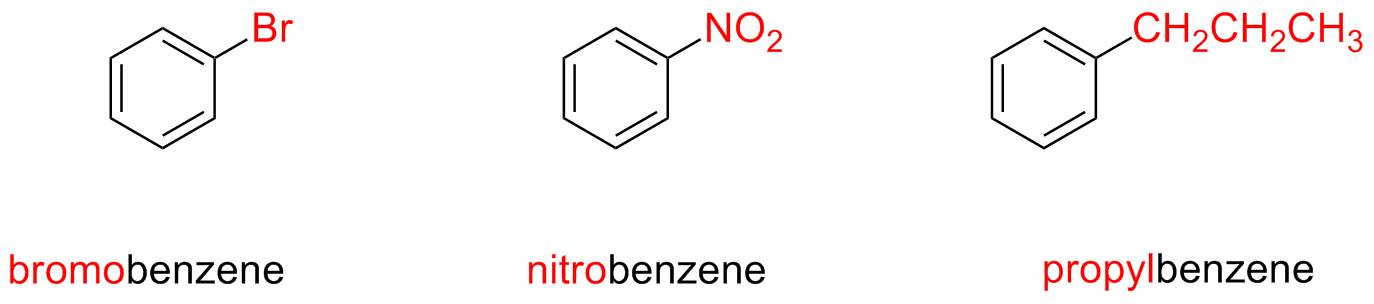
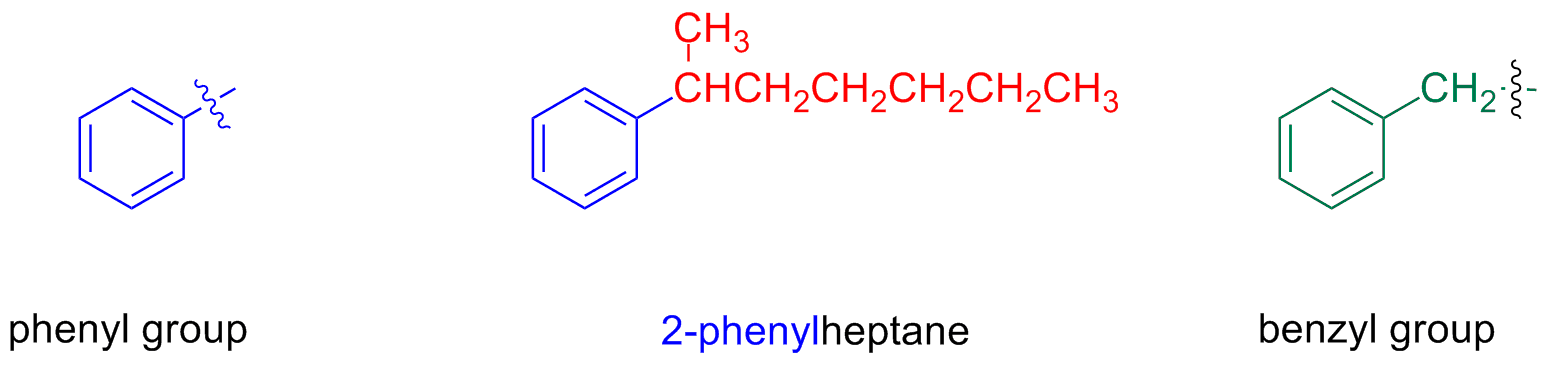
If the alkyl substituent has a smallest or equal number of carbon atoms as the ring, the compound is named as an aromatic compound bearing an alkyl substituent
If the alkyl substituent has a greatest number of carbon atoms than the ring, the compound is named as an alkyl compound bearing an phenyl substituent
The name phenyl indicates –C6H5, when it is a substituent
The group C6H5CH2- is named benzyl
Disubstituted Benzenes: orto / meta / para nomenclature
Disubstituted Benzenes: orto / meta / para nomenclature
Disubstituted benzenes are named with the prefixes:
orto- (o), meta- (m), para- (p)
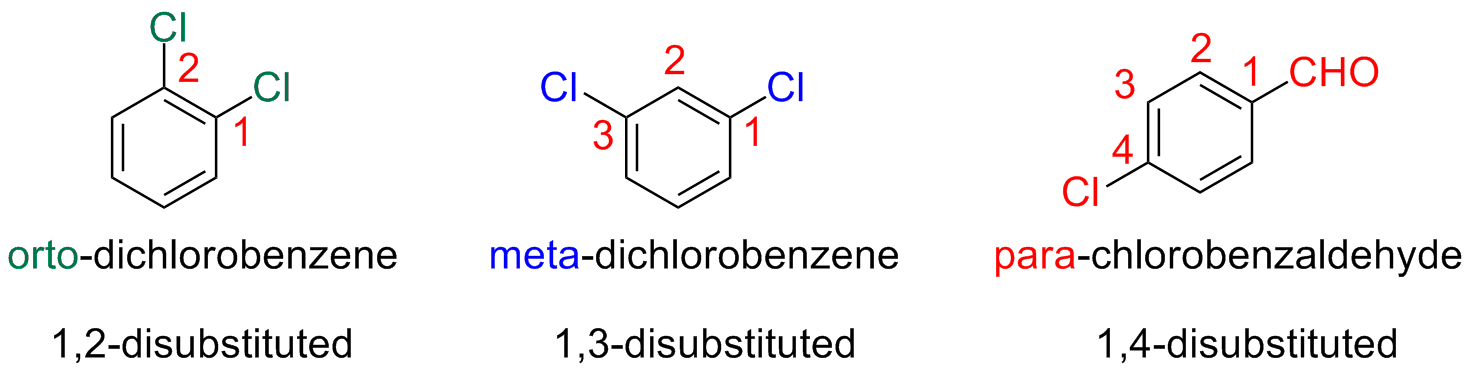
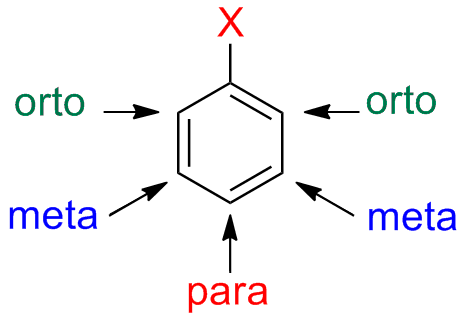
The nomenclature based on the prefixes orto-, meta-, para- is useful when, in considering reactions, one need to indicate where the reaction will occur (regioselectivity)
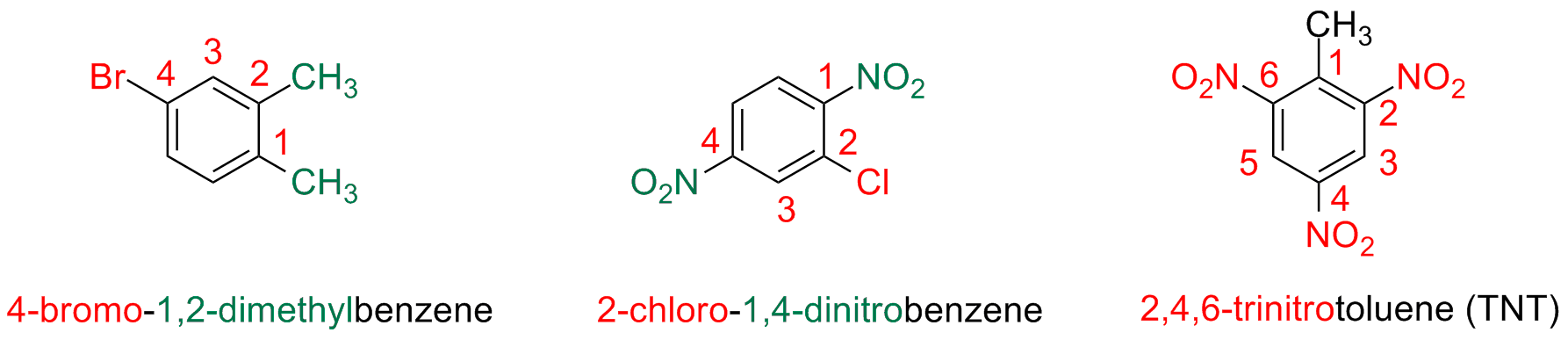
Polysusbstituted Benzenes
Polysusbstituted Benzenes
Benzene derivatives with more than two substituents are named by numbering the position of each substituent using the
numbers as low as possible. Substituents are indicated in alphabetic order.
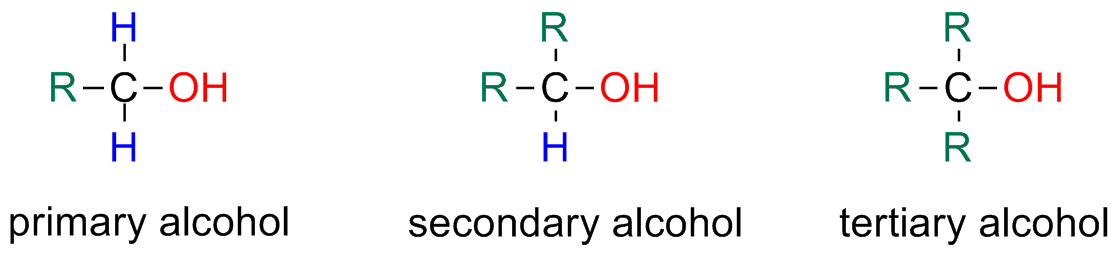
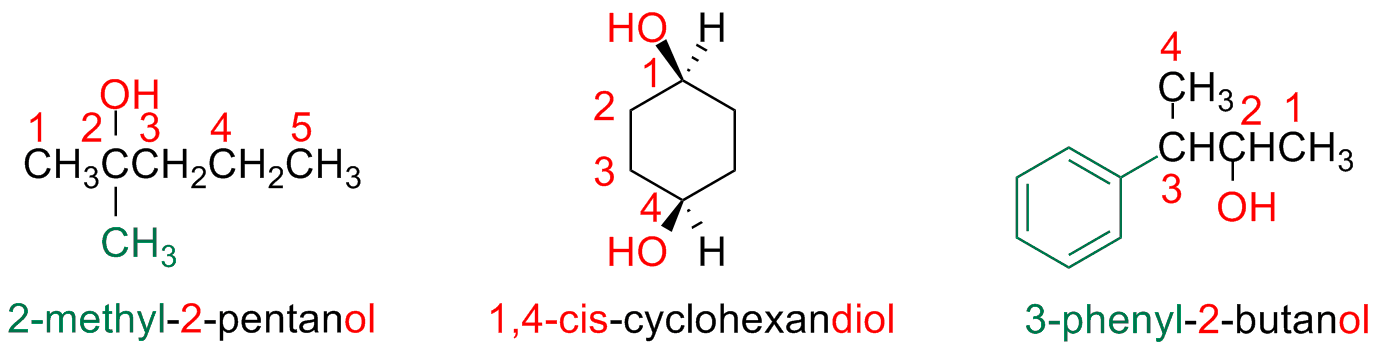
Alcohols
Alcohol Classification: primary / secondary / tertiary
Alcohol Classification: primary / secondary / tertiary
Alcohols can be classified as primary, secondary or tertiary
Step 1 - Identify the parent hydrocarbon chain
Step 1 - Identify the parent hydrocarbon chain
The main chain is the longest hydrocarbon chain that contains the hydroxyl group
the suffix is -ol
Step 2 - Number the parent chain carbon atoms
Step 2 - Number the parent chain carbon atoms
The parent chain carbon atoms are numbered starting from the edge closest to the hydroxyl group
Step 3 - Write the name as a single word
Step 3 - Write the name as a single word
Number the substituent based on their position on the parent chain and write the name positioning them in alphabetic order
Ethers and Epoxydes
Simple common Ethers
Simple common Ethers
Simple Etehrs, not having any other functional groups are named on the base of the organic susbtitutents and by adding the word ether
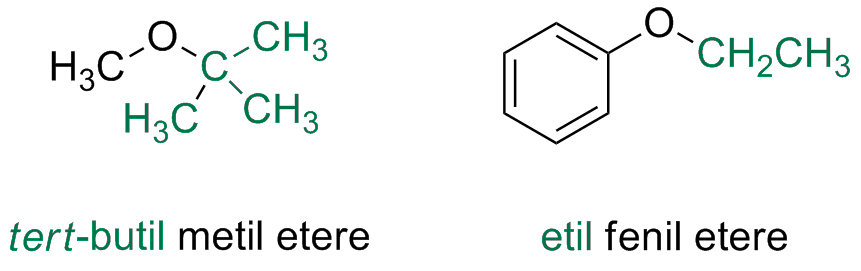
Complex Ethers
Complex Ethers
If other functional groups are present, the ether is always considered an alkoxy substituent
Aldehydes and Ketones
Aldehydes
Aldehydes
The aldehydes are named by substituting the final vocal of the parent alkane with the suffix -al
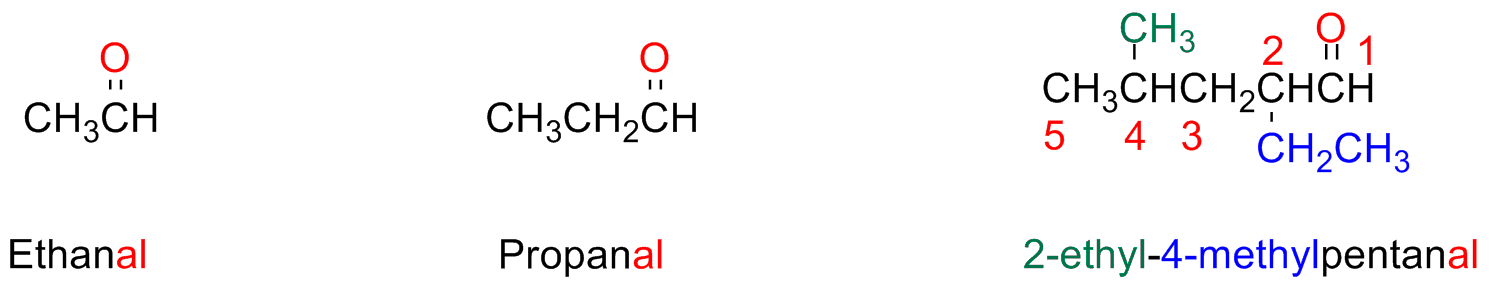
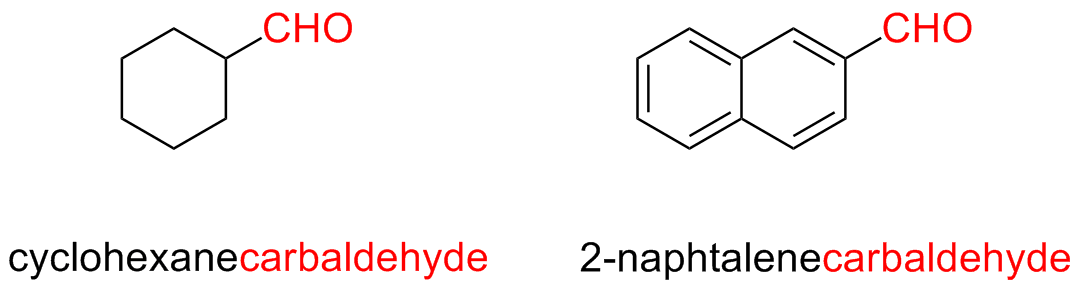
The parent chain must contain the –CHO group and this carbon atom takes the number 1
For more compelx aldehydes, where the –CHO group is linked directly to a ring, the suffix –carbaldehyde is used
Ketones
Ketones
The ketones are named by substituting the final vocal of the parent alkane with the suffix -one
The parent chain must contain the ketone group and is numbered from the edge closest to the ketone
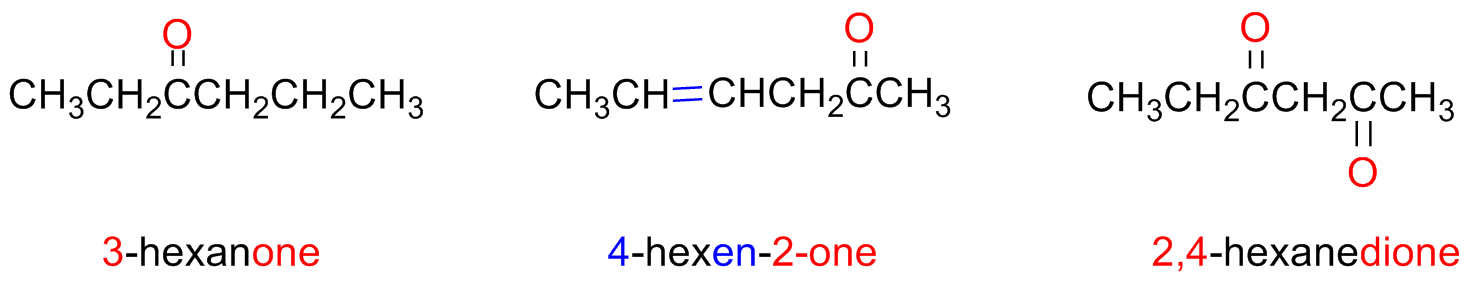
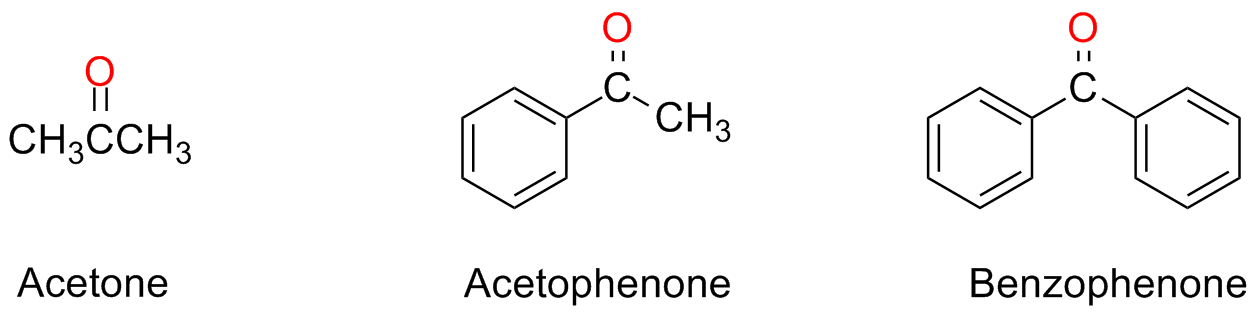
Some ketones have a trivial name
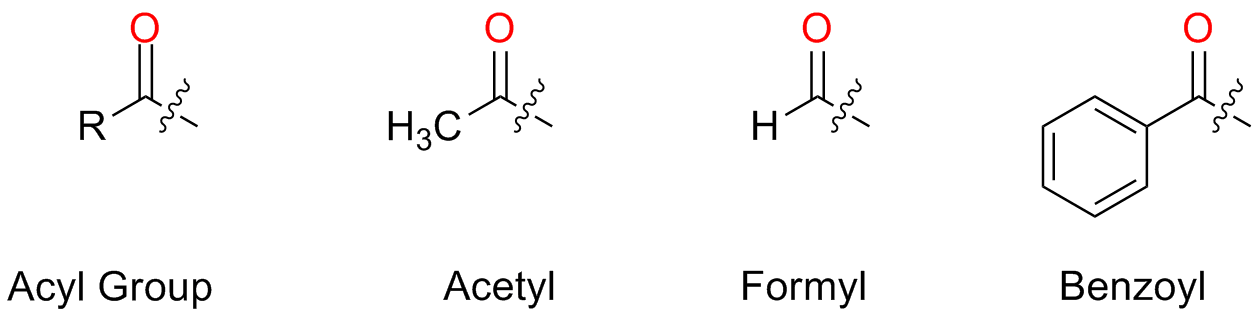
When the R-C=O groups is taken as a substituent, the name corresponding to the acyl group is used and the suffix -yl is added to the substituent
The name is taken from the parent carboxylic acid and the suffix is changed from -oic to -yl
If therea other functional groups and the ketone is taken as a substituent, it is named by using the prefix "oxo"
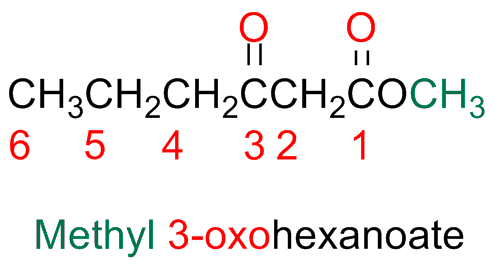
Carboxylic Acids and their Derivatives
Carboxylic Acids
Carboxylic Acids
There are two types of Nomenclature based on the complexity of the acid
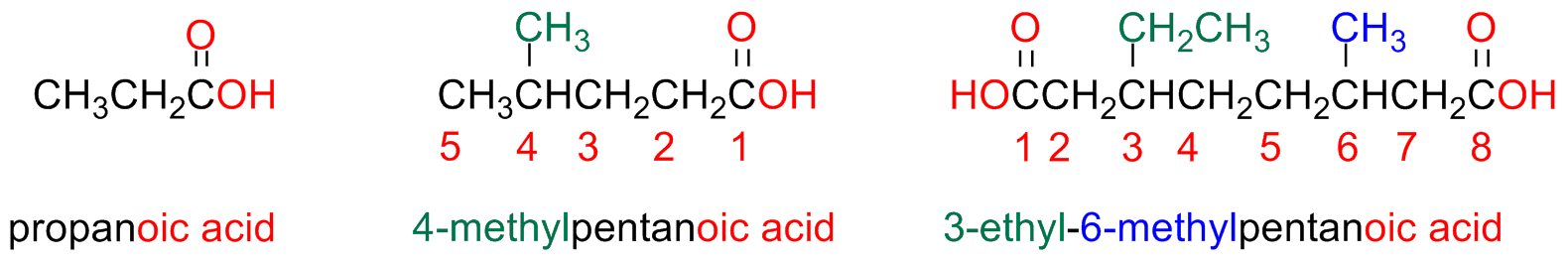
Straight chain acids are named by substituting the suffix -e of the parent alkane -oic followed by the word acid
The carboxyl carbon atom is always the first atom in the numbering sequence
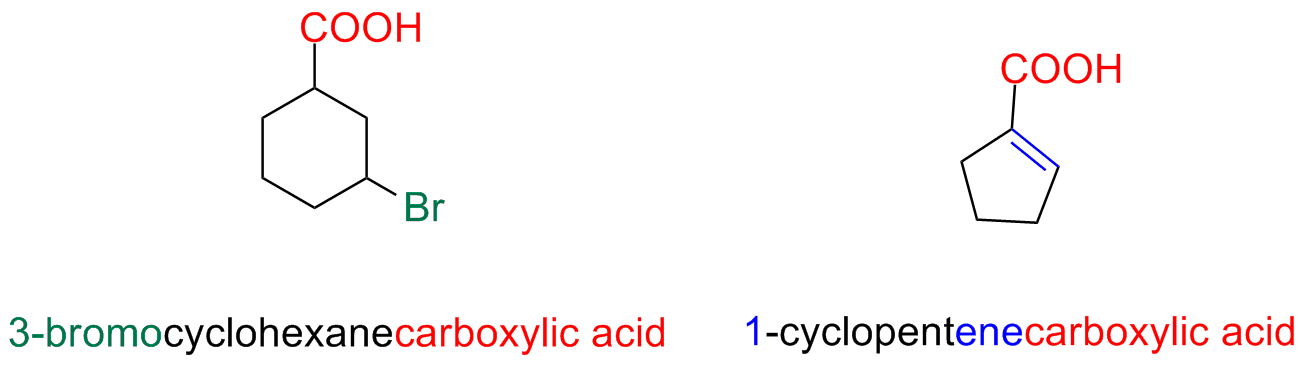
When the carboxyl is linked to a ring, the suffix is -carboxylic followed by the word acid
There are several other trivial names that are commonly used
Nitriles
Nitriles
The compounds characterized by the presence of the -C≡ N functional group are named nitriles
They are related to the carboxylic acid by a similar reactivity
Their name is obtained by adding the suffix –nitrile to the name of the parent alkane and the carbon of the nitrile takes the number 1
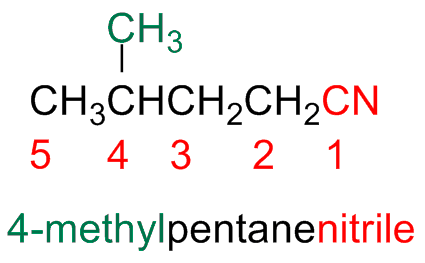
The names of more complex nitriles are obtained by substituting the suffix -oic of the parent acid with the suffix -nitrile
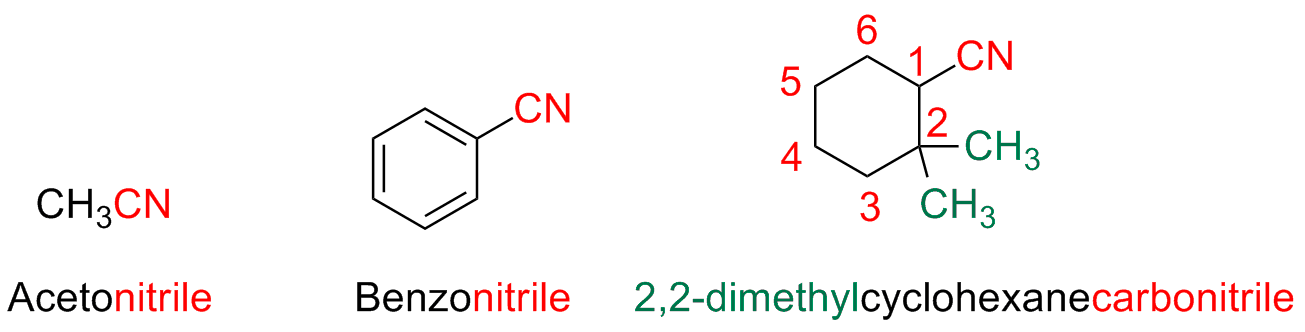
When the –CN group is directly linked to a ring, the suffix -carboxylic of the parent acid is substituted with the suffix -carbonitrile
Acyl Halides
Acyl Halides
Acyl halides are named by writing the name of the acyl group followed by the name of the halide
The name of the acyl group comes from the name of the parent carboxylic acid and substituting the suffix -ic with the suffix -yl or -carboxylic with -carbonyl
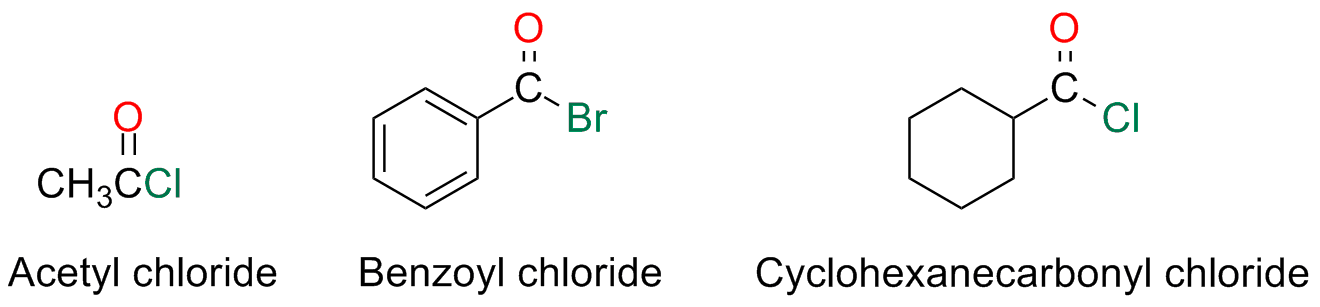
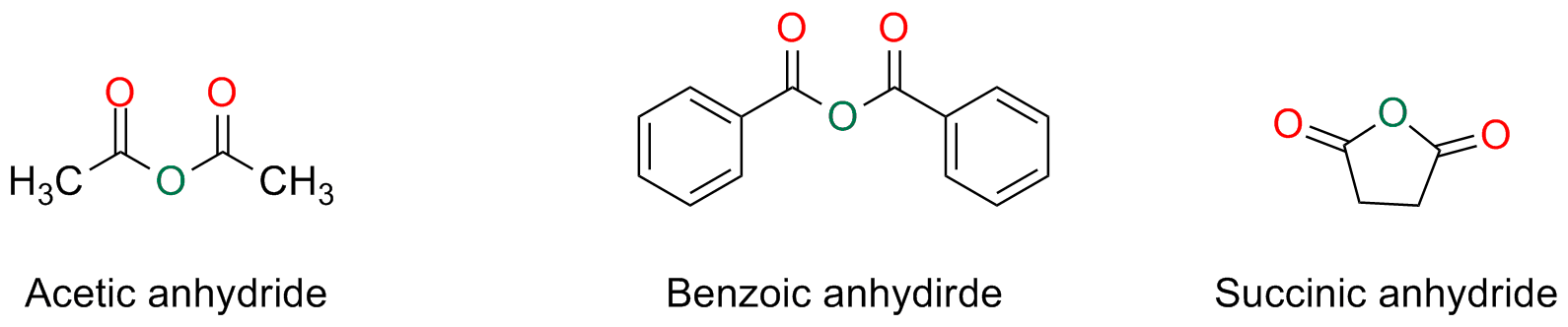
Anhydrides
Anhydrides
Symmetric anhydrides, derived from monocarboxylic acids, and the cyclic ones, derived by dicarboxylic acids are named by substituting the word acid with the word anhydride
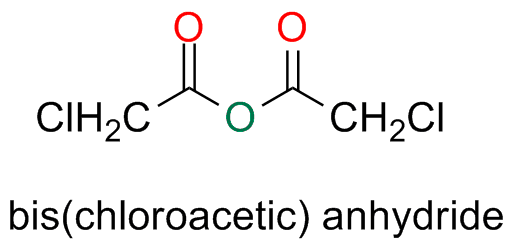
Symmetric anhydrides, derived from substituted monocarboxylic acids are named by adding the prefix bis- to the name of the acid
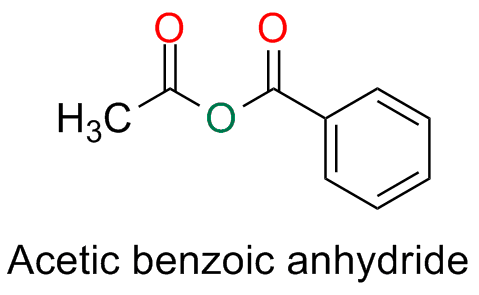
Unsymmetrical anhydrides derived from two different monocarboxylic acids are named by writing the names of the two acids in alphabetic order
Esters
Esters
The name of esters and salt of carboxylic acids is obtained in the same way
The suffix -ic is substituted by the suffix -ate and the name of the alkyl group (ester) or the cation (salt) is preceding the whole name
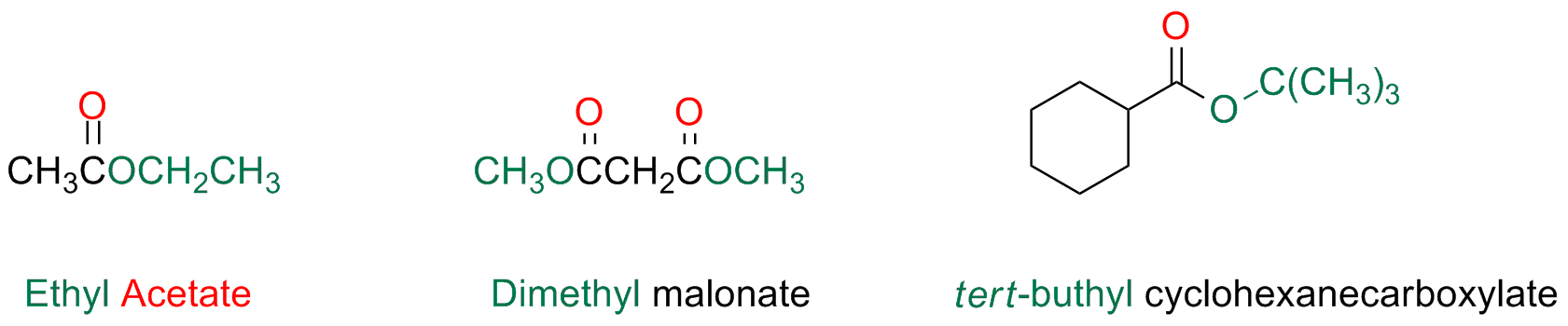
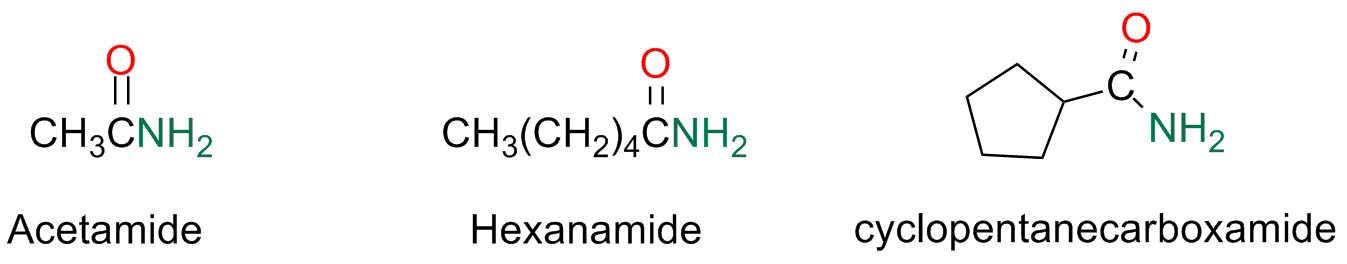
Amides
Amides
The amides that do not have any susbstituent on their nitrogen atom are named by substituting the suffix -ic or -oic of the parent acid with -amide or -carboxylic with -carboxamide
If the amide nitrogen atom bears alkyl / aryl substituents, the alkyl / aryl substituent is placed before the name of the amide and its position as substituent is indicated by a capital N in italic.
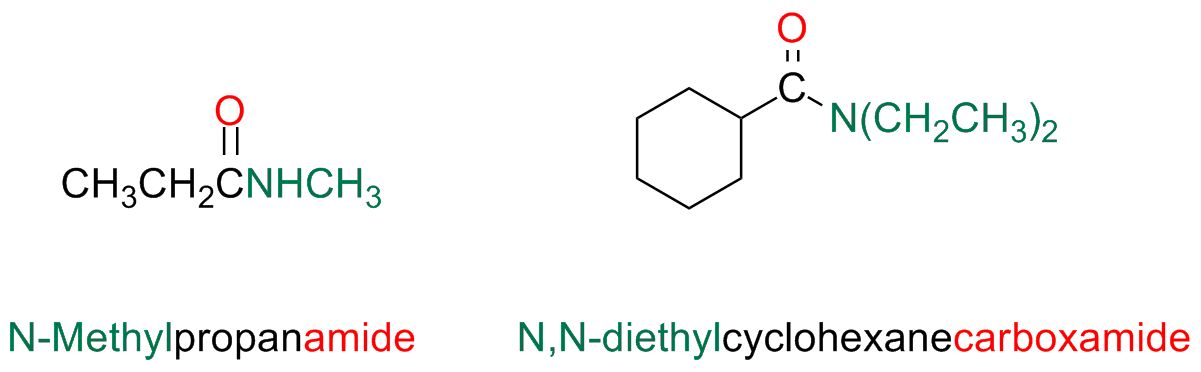
Amines
Amines
Amines
Amines can be substituted with alkyl (alkylamines) or aryl (arylamines) groups
The chemistry of these two classes is similar but shows sometimes a few differences
Amines: Primary (RNH2) Secondary (R2NH) Tertiary (R3N)
The terms primary, secondary and tertiary are used in a different way with respect to other compoudns (i.e. alcohols)
Here these terms indicate the degree of substitution of the Nitrogen atom
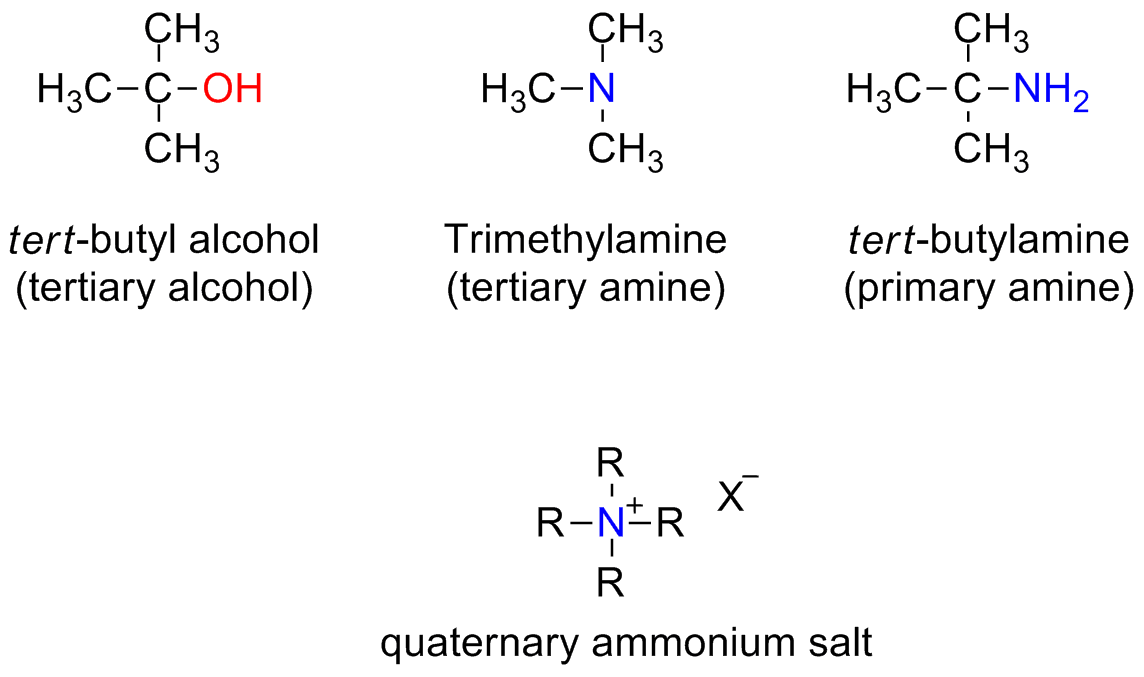
There are also compounds bearing four substituents on the nitrogen. The nitrogen bear also a positive charge. Those compounds are called quaternary ammonium salts
IUPAC Nomenclature of the Amines
IUPAC Nomenclature of the Amines
In the IUPAC Nomenclature system the primary amines are named in different ways
Simple amines are named by adding the suffix -amine to the name of the alkyl substituent
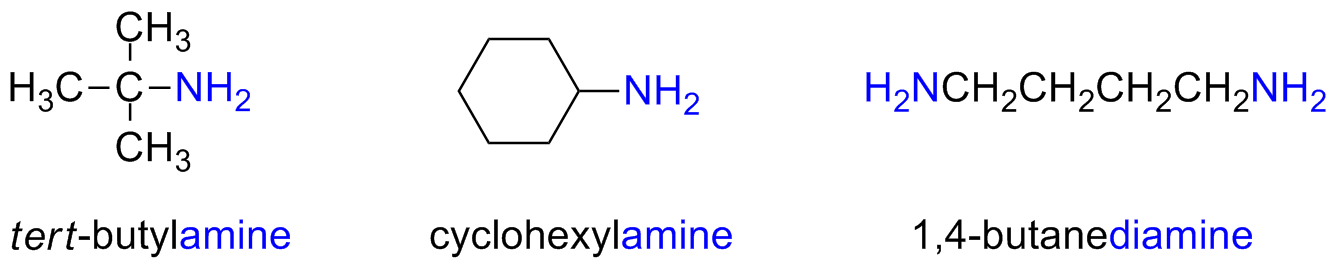
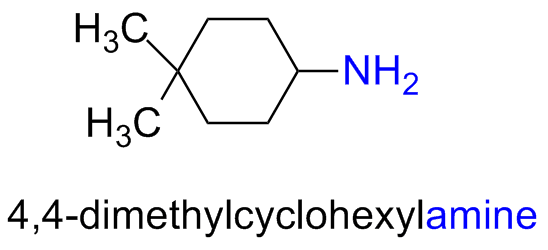
Alternatively, the suffix -amine can be added in place of the final -e of the parent compound
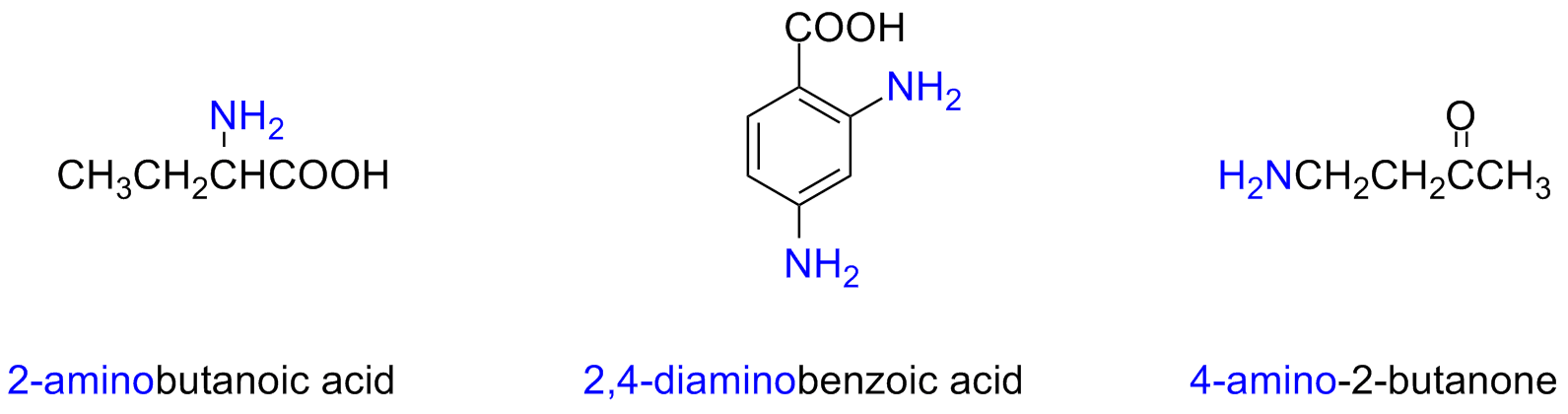
When the molecule has more than a functional group the -NH2 is considered an amine substituent of the base molecule
The name of secondary and tertiary symmetric amines is obtained by adding the prefix di- or tri- to the alkyl / aryl substituent 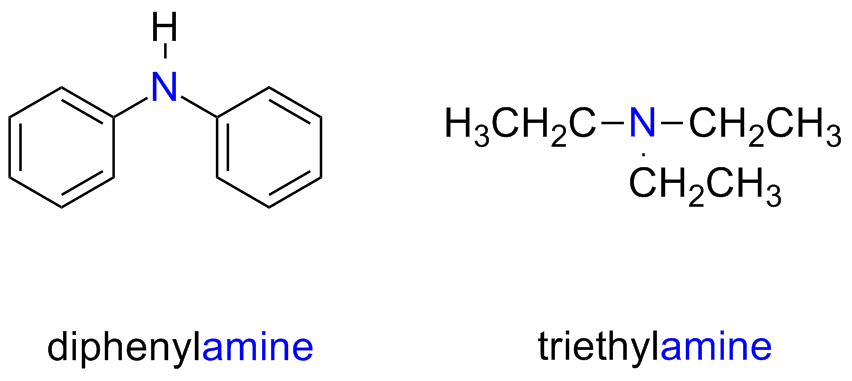
Secondary and tertiary unsymmetric amines are named as N-substituted primary amines
The longest alkyl group is taken as the main chain for reference name, while the other two alkyl groups are N-substituents
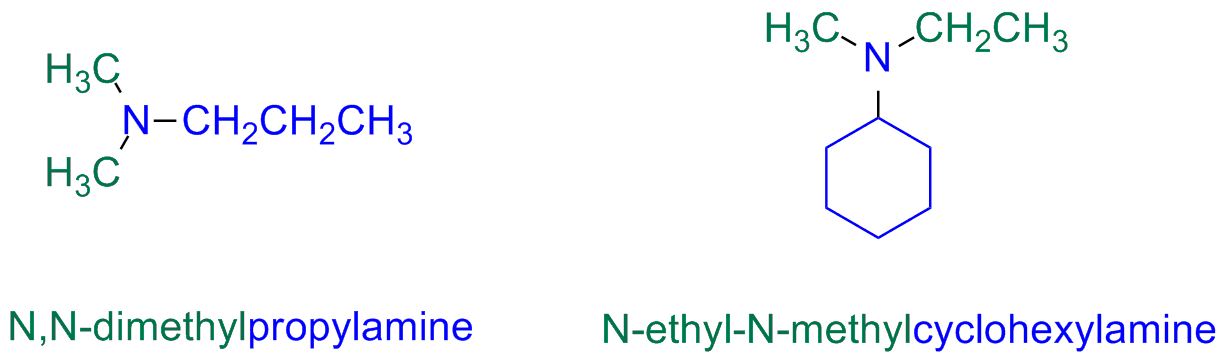
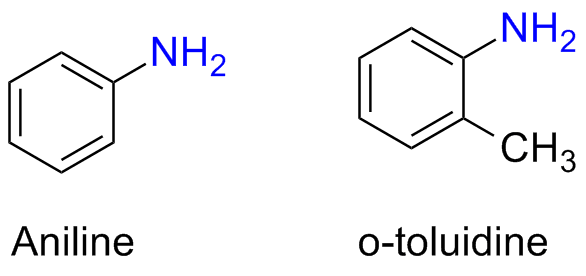
There only few trivial names for alkylamine but there are several for arylamines
as an example, very well known amines are aniline and toluidine
Heterocyclic Amines
Heterocyclic Amines
Heterocyclic amines: compounds in which the nitrogen atom is part of a ring
In the numbering scheme, the nitrogen (at least one of them) bears the number 1